Details of the Drug
General Information of Drug (ID: DM9JON3)
| Drug Name |
SU5402
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
su5402; 215543-92-3; SU 5402; SU-5402; 3-[3-(2-Carboxyethyl)-4-methylpyrrol-2-methylidenyl]-2-indolinone; (Z)-3-(4-methyl-2-((2-oxoindolin-3-ylidene)methyl)-1H-pyrrol-3-yl)propanoic acid; CHEMBL89363; 3-[(3-(2-CARBOXYETHYL)-4-METHYLPYRROL-2-YL)METHYLENE]-2-INDOLINONE; J-502595; 3-{[3-(2-carboxyethyl)-4-methylpyrrol-2-yl]methylene}-2-indolinone; 3-[4-methyl-2-[(Z)-(2-oxo-1H-indol-3-ylidene)methyl]-1H-pyrrol-3-yl]propanoic acid; (Z)-3-(4-Methyl-2-((2-oxoindolin-3-ylidene)-methyl)-1H-pyrrol-3-yl)propanoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
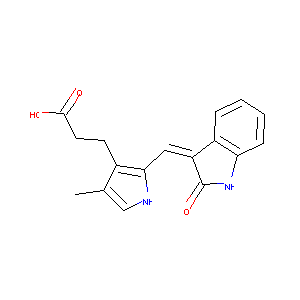 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 296.32 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Multiple myeloma | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A83 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
